NEWSROOM
-
Fudan University Team Led by Sheng Weiqi and Xu Midie Reports CCT6A Modulates Tumor Microenvironment via Intercellular Shuttling, Establishing a Pro-Tumor "Metabolic Niche"
Gastric cancer (GC) ranks as the fifth most common malignancy globally and is the second leading cause of cancer-related deaths, with over 1 million new cases and approximately 770,000 deaths annually. Despite advances in treatment modalities such as targeted therapy and immunotherapy, chemotherapy remains the mainstay for locally advanced and metastatic GC. However, the development of chemoresistance significantly limits treatment efficacy, underscoring an urgent need to elucidate the mechanisms of resistance and develop novel therapeutic strategies.

On August 13, 2025, a team led by Sheng Weiqi and Xu Midie from Fudan University Shanghai Cancer Center published significant research in the journal Advanced Science (IF: 14.1), entitled "Exosomal CCT6A Secreted by Cancer-Associated Fibroblasts Interacts with β-Catenin to Enhance Chemoresistance and Tumorigenesis in Gastric Cancer". This study reveals that cancer-associated fibroblasts (CAFs) secrete the CCT6A protein via exosomes, modulating metabolic homeostasis within the tumor microenvironment (TME) to establish a "metabolic niche" conducive to tumor progression and chemoresistance, thereby offering a new therapeutic target for GC.
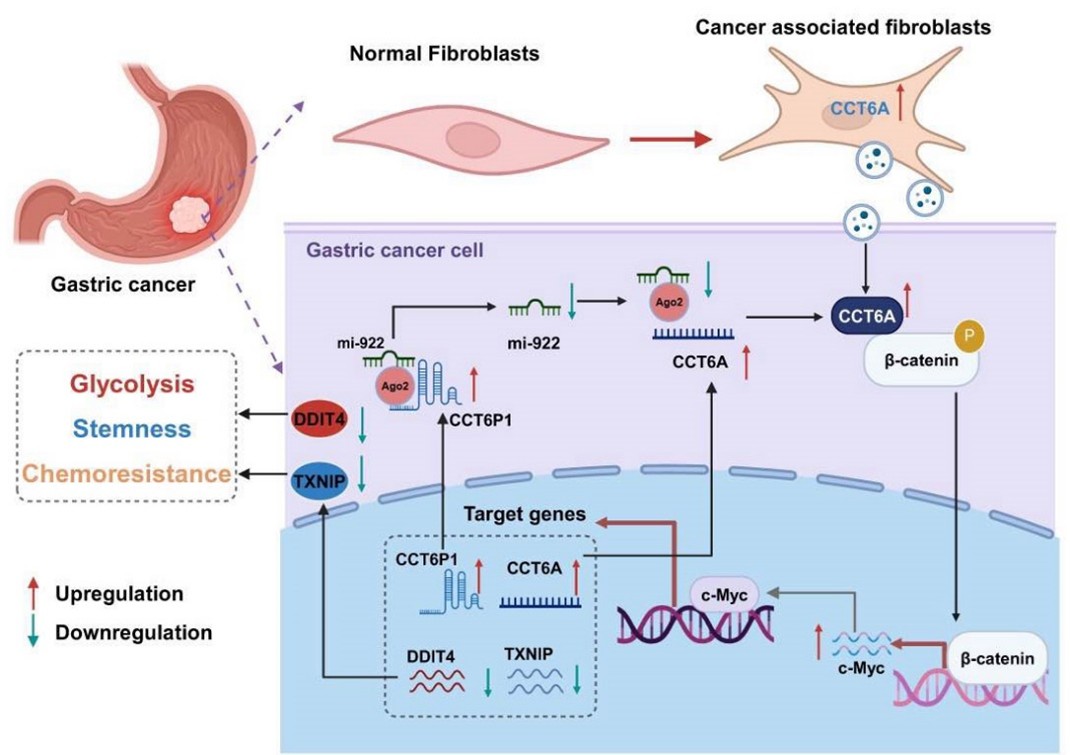
CAFs are key components of the TME and can drive GC progression and chemoresistance. This study is the first to demonstrate that CCT6A drives the proliferation of CAFs in the GC microenvironment and provides energetic support for CCT6A secretion via exosomes by enhancing their glycolytic capacity. Exosome-derived CCT6A subsequently shuttles to GC cells, activating the CCT6A/β-catenin/c-Myc signaling axis: CCT6A binds to β-catenin, promoting its nuclear translocation and driving c-Myc transcriptional activity. This, in turn, suppresses the expression of metabolic regulators DDIT4 and TXNIP, remodels the GC metabolic microenvironment, and ultimately promotes cancer stemness, chemoresistance, and malignant progression. Furthermore, the study discovered that CCT6A forms a positive feedback loop with its pseudogene, CCT6P1: CCT6P1 stabilizes CCT6A expression by sponging miR-922, while c-Myc simultaneously activates the transcription of both, creating a self-reinforcing pro-tumor network. Animal experiments confirmed that targeting β-catenin or c-Myc significantly inhibited tumor growth, while overexpression of DDIT4/TXNIP could reverse the tumor-promoting effects of CCT6A. These findings not only reveal the crucial mediating role of CCT6A in coordinating GC stemness, chemoresistance, and metabolic reprogramming but also highlight its dual value as a diagnostic biomarker and therapeutic target.
The core findings of this study are: (1) High expression of CCT6A in CAFs correlates positively with GC progression and plays a key role in tumor stemness, chemoresistance, and glycolytic regulation; (2) CCT6A is primarily transferred from CAFs to tumor cells via exosomal transport; (3) CCT6A activates the β-catenin/c-Myc/DDIT4-TXNIP signaling axis; (4) The CCT6A pseudogene CCT6P1 acts as a competing endogenous RNA (ceRNA) to stabilize CCT6A expression by sponging miR-922, (5) while c-Myc concurrently activates the transcription of both CCT6A and CCT6P1, thereby amplifying the oncogenic signal. Together, these findings establish CCT6A as a potential target for CAF-directed intervention in GC therapy.
In conclusion, this study holds significant clinical translational value: The findings suggest that serum CCT6A levels could serve as a non-invasive biomarker for early diagnosis and recurrence/metastasis of GC. Targeting the CCT6A/β-catenin/c-Myc signaling axis or combining it with metabolic intervention holds promise as a novel strategy to overcome chemoresistance. This research innovatively reveals the mechanism by which CAFs remodel the tumor metabolic microenvironment via exosomal delivery of CCT6A, providing a new target for precision therapy in GC. The future development of CCT6A inhibitors or combination therapies involving metabolic modulators may break through the current bottlenecks in GC treatment. This study elucidates the key mechanisms of metabolic reprogramming in the TME from a novel perspective, demonstrating how exosome-mediated formation of a "metabolic niche" promotes tumor drug resistance, thus pointing the way for clinical translational research. We look forward to future translational studies that will advance this discovery into clinical practice!