NEWSROOM
-
A Multi-Cohort Analysis from "Fudan University Shanghai Cancer Center" Reveals Global Patterns in Genomic Mutations among Patients with Early-Onset Colorectal Cancer
July 2, 2025 - A study led by Professor Li Xinxiang (Director) and Professor Ma Yanlei (Deputy Director) from the Department of Colorectal Surgery at our hospital has, for the first time, systematically revealed genomic differences between early-onset colorectal cancer (diagnosis age < 50 years, EOCRC) and late-onset colorectal cancer (diagnosis age ≥ 50 years, LOCRC) stratified by tumor mutational burden (TMB). This study not only provides critical insights into the global rise in EOCRC incidence, but also establishes a scientific foundation for clinical personalized treatment strategies. The findings were published in The Lancet Oncology (IF = 35.9).
Colorectal cancer (CRC) affecting younger populations is a globally recognized concern. In recent years, the incidence of CRC among young adults has surged significantly, often accompanied by more aggressive clinical features such as higher rates of metastatic disease at diagnosis and poorer tumor differentiation. While prior studies suggested potential roles of genetic and environmental factors, the molecular mechanisms remained under-investigated. To address this, the research team organized a collaboration among leading institutions from China, the United States, Spain, and Qatar, including our hospital, Mayo Comprehensive Cancer Center (USA), Qilu Hospital of Shandong University, Vanderbilt University Medical Center, The First Affiliated Hospital of Zhejiang University College of Medicine, National Institute of Health/National Cancer Institute (USA), Institute of Biomedical Research of Salamanca (Spain), andsSidra Medicine (Qatar). The study integrated whole-exome sequencing and clinical targeted sequencing data from 17,133 CRC patients across eight countries spanning four continents, categorizing them into hypermutated (TMB > 15) and non-hypermutated (TMB ≤ 15) subgroups for systematic comparison of mutation patterns.
The study identified a unique TMB distribution pattern in EOCRC (Figure 1). Specifically, within the hypermutated subgroup, TMB was significantly higher in EOCRC than in LOCRC (Figure 1B, mean ratio 1.11). Conversely, in the non-hypermutated subgroup, EOCRC exhibited lower TMB (Figure 1C, mean ratio 2.92). Further analysis revealed that hypermutated EOCRC showed significantly elevated mutation frequencies in 25 key genes - including APC (75% vs. 58.6%), KRAS (53.3% vs. 32%), and CTNNB1 (31.6% vs. 18%) - whose aberrant activation may directly drive early tumorigenesis and rapid progression. In contrast, only BRAF and RNF43 mutations were less frequent in EOCRC, with odds ratios (ORs) predominantly distributed on the right side of the forest plot (Figure 1D). For non-hypermutated CRC, 9 genes had reduced mutation frequencies in early-onset cases, while only TP53 mutations increased, with ORs concentrated on the left side of the forest plot (Figure 1E). Professor Ma Yanlei noted: "These results demonstrate a bimodal distribution of mutation frequencies in EOCRC based on TMB stratification, highlighting distinct molecular characteristics between young and elderly patients. Our team introduced a novel analytical approach - calculating mutation rate to mitigate confounding effects from multiple mutations in single genes - further validating the reliability of our conclusions."
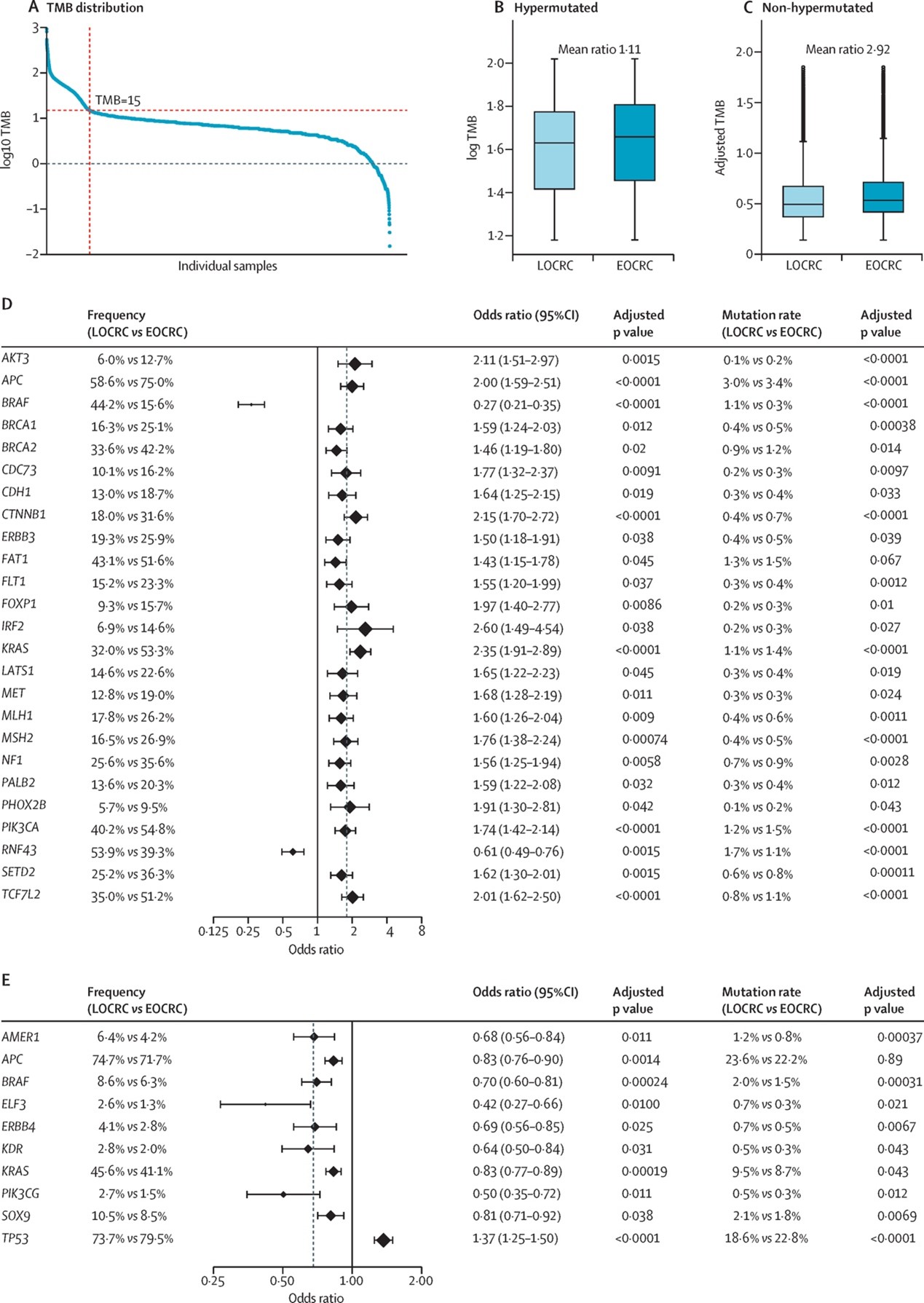
Figure 1
Additionally, the study found that mutation patterns in core driver genes (e.g., APC, KRAS, PIK3CA) were highly consistent globally in hypermutated EOCRC. The low frequency of BRAF mutations was universally observed across countries in EOCRC, while mutations in genes such as MTOR and ERBB3 exhibited significant heterogeneity depending on national or ethnic backgrounds, revealing country-specific mutation frequencies in young versus elderly CRC patients.
Notably, the team identified clinically relevant hotspot mutation differences. For example, BRAF V600E mutations were highly enriched in LOCRC (2.1% vs. 33.7%), whereas KRAS G12D mutations increased significantly in hypermutated EOCRC (17.4% vs. 8.3%). This finding offers new insights for targeted therapy. Furthermore, these molecular differences demonstrated cross-ethnic and cross-regional consistency: elevated TMB in hypermutated EOCRC was consistent in Asian and White populations, while enrichment patterns of APC and KRAS mutations were highly similar globally, underscoring the universality of these molecular features.
This study is the first globally to delineate the unique molecular landscape of EOCRC stratified by TMB, providing critical implications for clinical practice. Hypermutated EOCRC patients may exhibit heightened sensitivity to immune checkpoint inhibitors, independent of microsatellite stable (MSS)/mismatch repair proficient (pMMR) status. Moreover, enrichment of KRAS G12D mutations identifies potential candidates for targeted drug clinical trials.
The team acknowledged limitations and future directions: (1) Samples were predominantly from large medical centers in middle-to-high-income countries, with limited data from Africa and South America, restricting generalizability; (2) Lack of key clinical information (e.g., germline mutations, MSI status, treatment history) may impact clinical interpretation; (3) Inconsistent sequencing platforms and definitions of "hypermutated" may introduce bias.
Nevertheless, Professor Ma Yanlei emphasized: "This study advances our understanding of why CRC is increasingly affecting young adults. Given the global concern, we call for enhanced international collaboration to establish standardized biobanks for young populations and integrate genomics with clinical data to ultimately achieve precise subtyping and personalized treatment." Currently, Ma’s team is further investigating the etiology of these mutation patterns and their prognostic implications to optimize patient management.
Professor Andreas Seeber from the Comprehensive Cancer Center at Medical University of Innsbruck and General Hospital of Oberwart commented: " This study represents a major step forward in characterising the molecular complexity of EOCRC. Using one of the largest aggregated sequencing datasets to date - integrating data from more than 17,000 CRC tumours across eight countries - the authors dissected reproducible genomic signals despite regional and technical heterogeneity. The finding that EOCRC - particularly hypermutated EOCRC - harbours higher frequencies of driver mutations such as APC, KRAS, and CTNNB1 than LOCRC, suggests a more accelerated mutational trajectory in younger patients (aged <50 years)." He stressed that the research confirms a pivotal concept: "EOCRC is not merely an age-shifted form of CRC but a biologically distinct disease requiring a tailored diagnostic and treatment management," warranting both global collaboration and local readiness to adopt precision oncology approaches.

